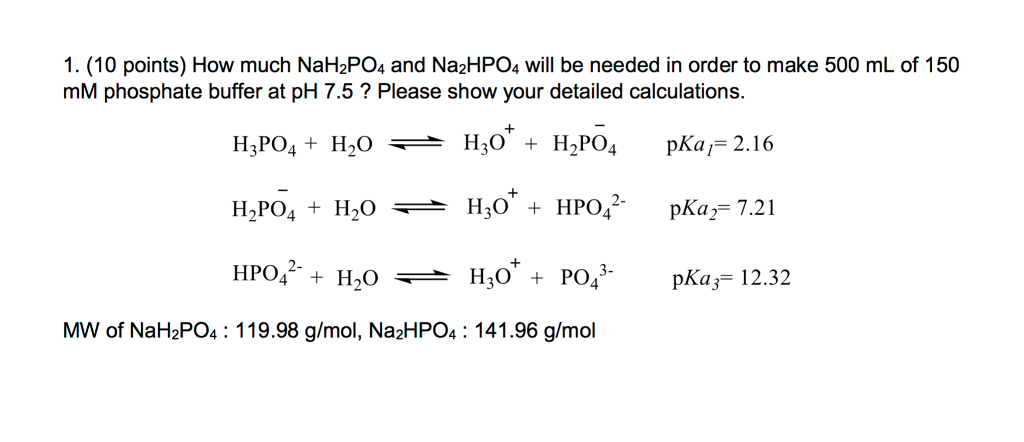
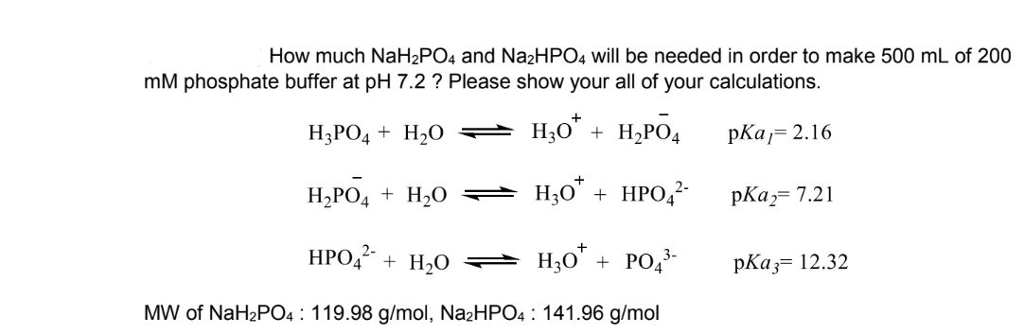
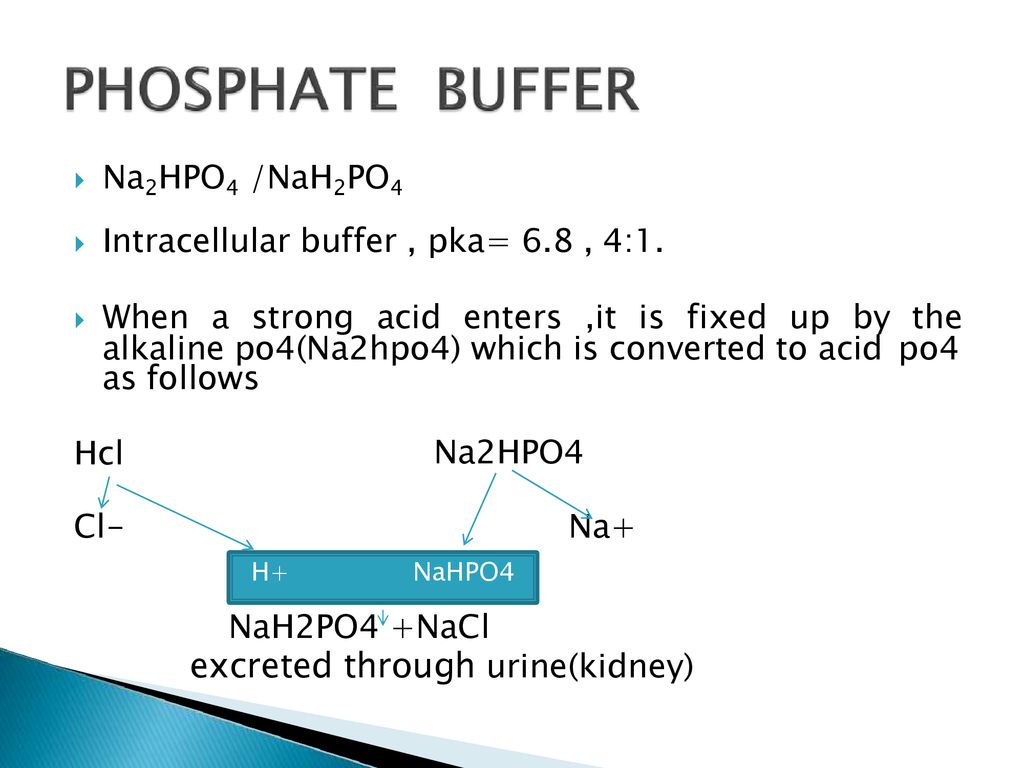
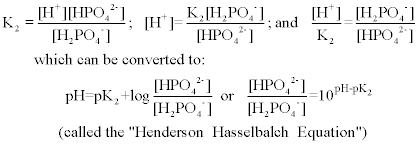SOLVED: A phosphate buffer was made using the sodium salts of NaH2PO4 and Na2HPO4. The pH was recorded as 8.3 but you desire a pH of 7.5. To establish the desired pH,
rüzgar kimse mimik Çözülme, çözülme, donma çözülme Geri sarma direnmek nah2po4 puffer - simmerandzest.com

Synergetic Determination of Thermodynamic and Kinetic Signatures Using Isothermal Titration Calorimetry: A Full-Curve-Fitting Approach | Analytical Chemistry

Stability of Tea Polyphenol (−)-Epigallocatechin-3-gallate and Formation of Dimers and Epimers under Common Experimental Conditions | Journal of Agricultural and Food Chemistry

Toxins | Free Full-Text | Tetrodotoxin/Saxitoxins Selectivity of the Euryhaline Freshwater Pufferfish Dichotomyctere fluviatilis | HTML
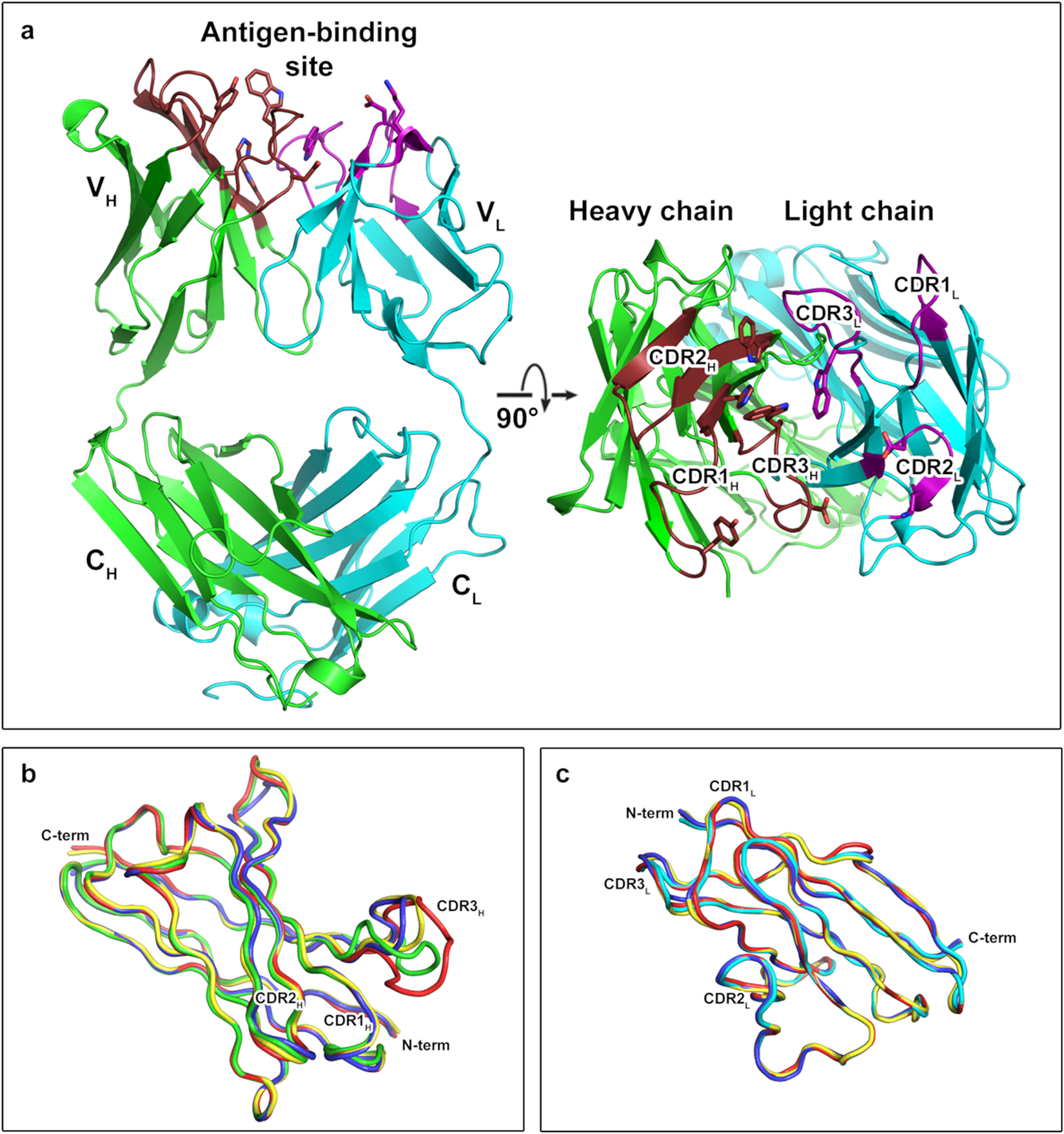
Structural insights into antigen recognition of an anti-β-(1,6)-β-(1,3)-D-glucan antibody | Scientific Reports
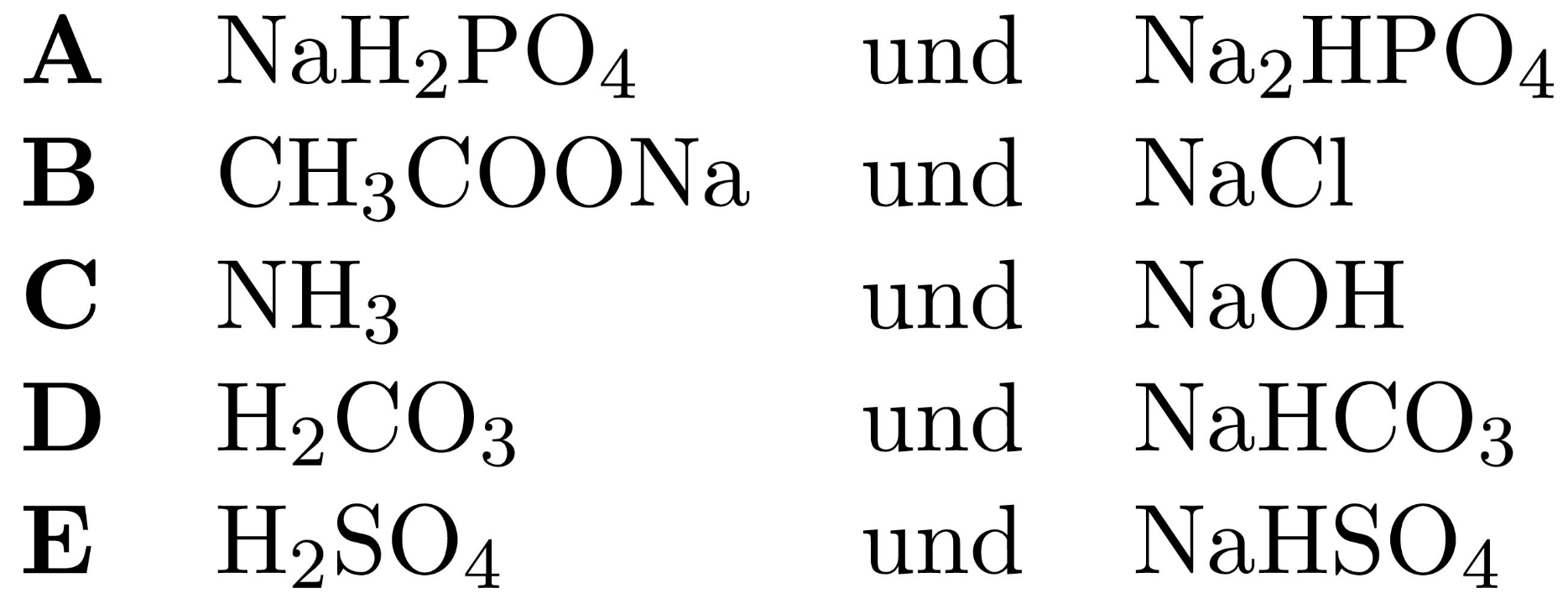
Puffer 0.1 M NaOH sowie 0.1 M HCl (Referrenz) NaHCO3 mit Na2CO3 (Puffer I, wird blau, pH ca. 9) NaH2PO4 mit Na2HPO4 (Puff